研究背景
錫基箔材負極具有容量高、成本低、加工容易等優(yōu)勢,是一種理想的高能量密度鋰離子電池負極材料。然而,錫箔負極面臨著循環(huán)過程中活性鋰損失多、活性材料/電解液之間副反應嚴重以及電極電化學反應不均勻等問題,導致了全電池實際能量密度低以及循環(huán)壽命差,限制了錫箔負極在鋰電池中應用。
因此,亟需開發(fā)簡單有效的方法解決以上問題,并且開展其在實際全電池中的應用研究。
成果簡介
近日,華中科技大學孫永明教授課題組在國際知名儲能期刊EnergyStorageMaterials上發(fā)表了題為“Heterogeneous Li-alloy interphase enabling Li compensation during cycling for highenergy density batteries”的研究論文。該論文提出通過在錫箔上構(gòu)建共形的異質(zhì)鋰合金界面(鋰化液態(tài)合金,Sn/Li-LA),可以有效避免電解液與活性鋰錫合金的直接接觸,抑制電極循環(huán)過程中電解液與活性物質(zhì)之間的副反應。
同時,該異質(zhì)合金界面高的離子電導率可以促進界面離子傳輸,均勻化電化學反應,能有效緩解電極破裂與粉化失效。更為重要的是,該異質(zhì)鋰合金界面具有略高于鋰錫合金的脫鋰電位,可以在電池長期循環(huán)過程中過電位增加時觸發(fā)鋰補償,延長全電池的循環(huán)壽命。
當其與面載量為15.3 mg/cm2的三元(NCM622)正極組裝全電池時,電池的容量可達180mAh/g-1,230次循環(huán)容量保持率為96%。而使用純錫箔負極的對比電池在230次循環(huán)后容量僅為22mAh/g-1。
該工作揭示了箔材電極在高比能鋰電池中應用的關(guān)鍵因素,即均勻化電化學反應、界面副反應抑制、界面離子傳輸及循環(huán)過程鋰補償,并提供了一種潛在實際可行的解決方案,為錫箔負極用于高比能鋰電池的研究提供了新的思路。
圖文解讀
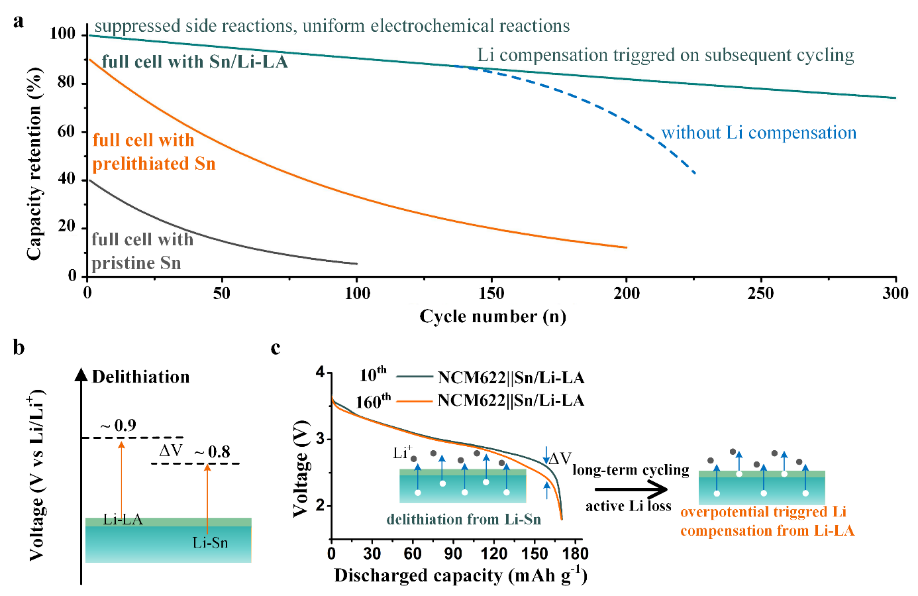
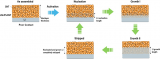
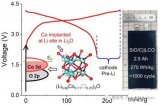
Fig. 1(a) Schematic of the capacity retention-cycle number curves of full cells paired with pristine Sn foil, prelithiated Sn foil and Sn/Li-LA foil. (b) Design principle of heterogeneous Li-alloy interphase. Delithiation voltage of the Li-LA should be slightly higher than the active Li-Sn alloy. (c) The comparison of voltage-capacity curves of the NCM622||Sn/Li-LA at the 10thand 160thcycles. The insets showed Li compensation from Li-LA would be triggered once the overpotential after long-term cycling increased to ΔV in full cells.
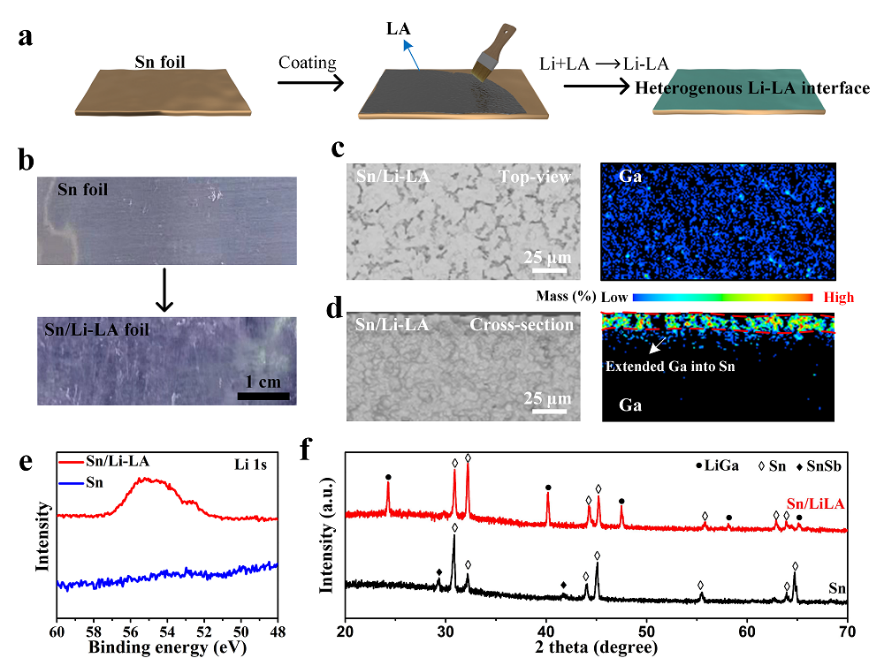
Fig. 2(a) Schematic of the preparation processes of the Sn/Li-LA foil utilizing the chemical lithiation reaction between the metallic Li foil and LA coating layer. (b) Digital photos of the Sn and Sn/Li-LA foils. (c, d) Top-view and cross-section EPMA images and the corresponding Ga element mapping images of the Sn/Li-LA foil, respectively. (e) High-resolution Li 1s XPS results of the Sn/Li-LA and pristine Sn electrodes. (f) XRD patterns of the Sn (with 5wt% Sb doped) and the Sn/Li-LA foils (Sn: JCPDS#04-0673, SnSb: JCPDS#33-0118, and LiGa: JCPDS #09-0043).
要點:
1.異質(zhì)鋰合金界面具有略高于鋰錫合金的去鋰化電位,可以在全電池過電位增加時,觸發(fā)鋰補償。
2.均勻的異質(zhì)鋰合金界面可以有效分隔電極活性材料與電解液的直接接觸,抑制他們之間的副反應。
3.異質(zhì)鋰合金界面主要成分為LiGa合金,其高的離子電導率能促進鋰離子在界面的快速傳輸。
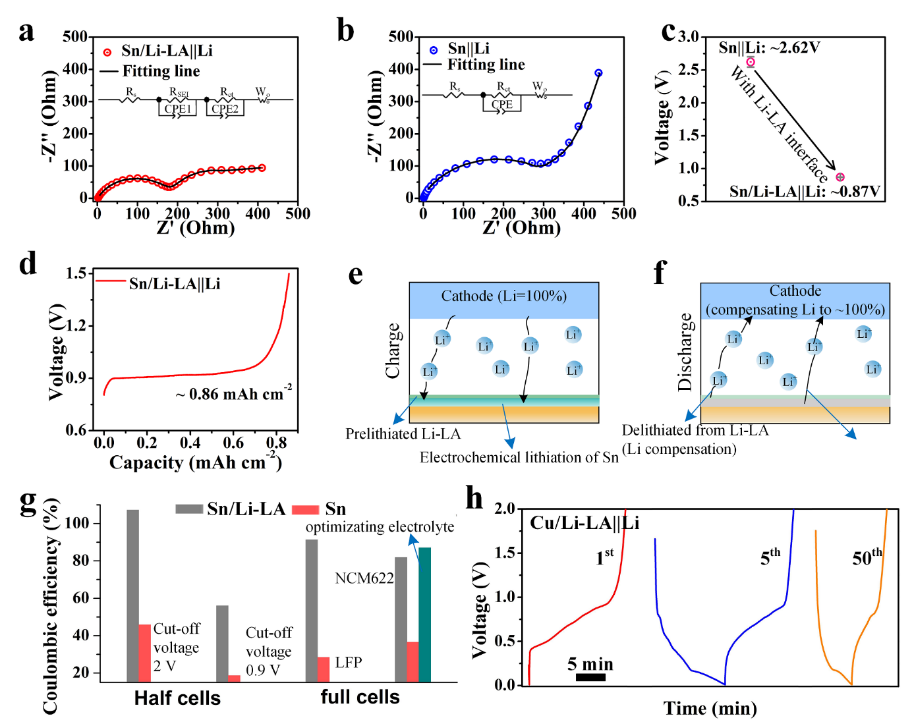
Fig. 3(a, b) Nyquist plots of the Sn/Li-LA||Li and Sn||Li cells before cycling, respectively. (c) Open-circuit voltages of the Sn/Li-LA||Li and Sn||Li cells. (d) Delithiation capacity of the Sn/Li-LA foil by charging Sn/Li-LA||Li cell to 1.5 V (vs. Li/Li+) at the current density of 0.1 mA cm–2. (e, f) Schematic of the electrochemical behavior of the Sn/Li-LA foil in full cell during the charge /discharge processes. (g) Comparisons of the initial Coulombic efficiency of Sn/Li-LA and Sn electrodes in half and full cells. (h) Charge/discharge curves of the Cu/Li-LA||Li cell for the 1st, 5th and 50thcycles.
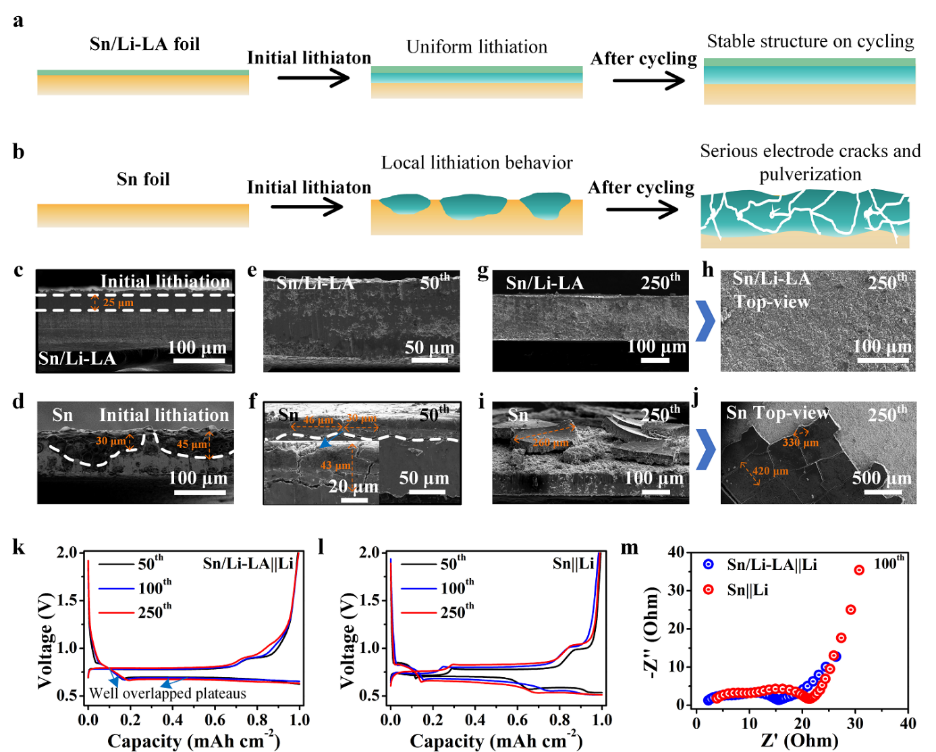
Fig. 4(a, b) Schematic of the structural evolutions of the Sn/Li-LA and Sn electrodes over lithiation/delithiation cycling. (c, d) Cross-section SEM images of the Sn/Li-LA and Sn electrodes after the initial lithiation at 1 mA cm–2for 3 hours, respectively. (e, f) Cross-section SEM images of the Sn/Li-LA and Sn electrodes after 50 cycles, respectively. (g, h) Cross-section SEM and top-view images of the Sn/Li-LA electrode after 250 cycles, respectively. (i, j) Cross-section SEM and top-view SEM images of the pristine Sn electrode after 250 cycles. (k, l) Voltage-capacity curves of the Sn/Li-LA and Sn electrodes at the 50th, 100thand 250thcycles, respectively. (m) Nyquist plots of the Sn/Li-LA||Li and Sn||Li cells after 100 cycles.
要點:
1.異質(zhì)鋰合金界面抑制了初始循環(huán)過程中電極界面副反應,減少了首次活性鋰損失,與純錫箔負極相比,首次庫倫效率得到了大幅度提升。
2.LiGa合金界面高的離子電導性可以有效均勻化電極的電化學反應,抑制電極的粉化,使Sn/Li-LA電極在循環(huán)過程中保持了良好的結(jié)構(gòu)完整性和穩(wěn)定性。
3.Sn/Li-LA電極充放電曲線在長循環(huán)過程中保持高度重合,進一步表明了其在循環(huán)過程中能夠保持電化學反應均勻性與電極結(jié)構(gòu)穩(wěn)定性。
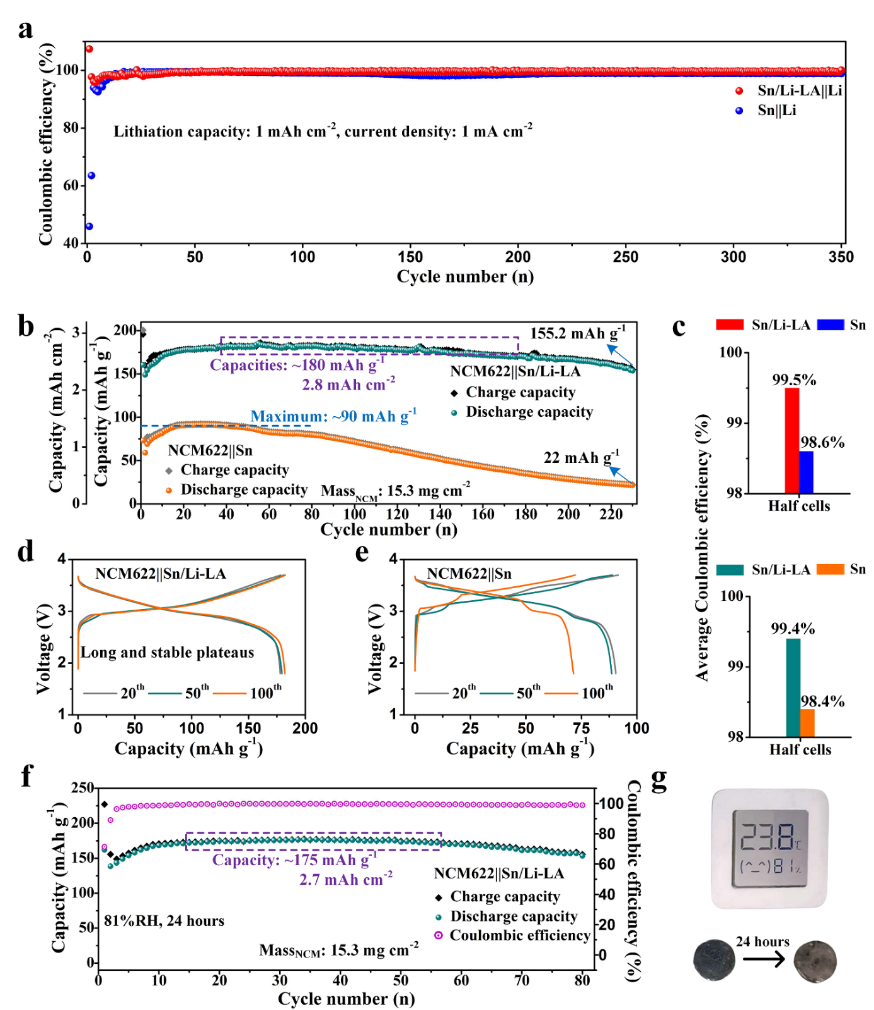
Fig. 5(a) Coulombic efficiency-cycle number plots of the Sn/Li-LA||Li and Sn||Li cells. (b)Electrochemical performance of NCM622||Sn/Li-LA and NCM622||Sn cells. (c) Average Coulombic efficiency of the Sn/Li-LA and Sn electrodes in half cell corresponding to (a) and full cell corresponding to (b), respectively. (d, e) Charge/discharge curves of NCM622||Sn/Li-LA and NCM622||Sn cells at the 20th, 50thand 100thcycles. (f) Electrochemical cycling of NCM622||Sn/Li-LA full cell using dual-salt electrolyte. The Sn/Li-LA electrode was exposed in ambient condition with the relative humidity of 81% for 24 hours before use. (g) The digital photo of the temperature and humidity indicator to show the test conditions and the corresponding digital photos of the electrodes before and after exposure.
要點:
1.在使用半電池和全電池進行長循環(huán)測試中,Sn/Li-LA電極比純錫電極展現(xiàn)出了更高的循環(huán)過程庫倫效率。
2.Sn/Li-LA負極搭配高載量NCM622(15.2 mg/cm2)正極組成的電池可以實現(xiàn)230次穩(wěn)定循環(huán),其可逆容量相較NCM622||Sn電池顯著增加。
3.Sn/Li-LA負極具有極好的空氣穩(wěn)定性,在大于80%的相對濕度下暴露24小時后仍能實現(xiàn)穩(wěn)定電化學充放電循環(huán)。
審核編輯:劉清
-
鋰電池
+關(guān)注
關(guān)注
260文章
8101瀏覽量
169954 -
充放電
+關(guān)注
關(guān)注
0文章
167瀏覽量
21840 -
電解液
+關(guān)注
關(guān)注
10文章
848瀏覽量
23094
原文標題:華中科技大學孫永明ESM:異質(zhì)鋰合金界面實現(xiàn)穩(wěn)定的鋰電錫箔負極
文章出處:【微信號:清新電源,微信公眾號:清新電源】歡迎添加關(guān)注!文章轉(zhuǎn)載請注明出處。
發(fā)布評論請先 登錄
相關(guān)推薦
多功能高熵合金納米層實現(xiàn)長壽命無負極鈉金屬電池

朗凱威即可探秘 12V 鋰電池電瓶:性能卓越的綠色能源

通過電荷分離型共價有機框架實現(xiàn)對鋰金屬電池固態(tài)電解質(zhì)界面的精準調(diào)控
全固態(tài)鋰金屬電池的鋰陽極夾層設計
石墨負極在鋰離子電池中的發(fā)展與儲鋰機制
鋰鐵電池和鋰電池的區(qū)別
為什么鋰電池受到擠壓重壓后會容易引起爆炸?

弱溶劑化少層碳界面實現(xiàn)硬碳負極的高首效和穩(wěn)定循環(huán)

topcon電池和異質(zhì)結(jié)電池區(qū)別
鋰電池和鉛酸電池有什么區(qū)別 鋰電池和鉛酸電池充電器通用嗎
磷酸鐵鋰電池和三元鋰電池熱穩(wěn)定性哪個更好?
通過金屬負極/LPSCl界面調(diào)控實現(xiàn)超穩(wěn)定全固態(tài)鋰金屬電池





 異質(zhì)鋰合金界面實現(xiàn)穩(wěn)定的鋰電錫箔負極
異質(zhì)鋰合金界面實現(xiàn)穩(wěn)定的鋰電錫箔負極











評論